Case study (29) – Acute Promyelocytic Leukemia (PML)
A 36-year-old woman has a history of fatigue for 1 week.
Her menstrual period has lasted 10 days, and she also noticed blood loss while brushing her teeth.
In the past day, she had a fever.
Laboratory Investigations:
Hemoglobin (Hb) 79 g/L
White blood cells (WBC) 3.2 X 109/L
Platelets 11 X 109/L
(PT) Prothrombin time 19 seconds (control 11–13 seconds)
(APTT) Activated partial thromboplastin time 64 seconds (control 30–40 seconds)
Thrombin time 28 seconds (control 18–20 seconds)
Fibrinogen 0.03 g/L (NR 0.2–0.4 g/L)
Fibrin degradation product (FDP) titer 1:60 (NR 2–4 g/L)
Questions:
Q1. What abnormalities are seen on the blood film and bone marrow?
Q2. What is the cause of abnormal coagulation?
Q3. What is the diagnosis?
Read alsoCase study (45) - Burkitt’s lymphoma
Q4. How is this condition managed?
Answers:
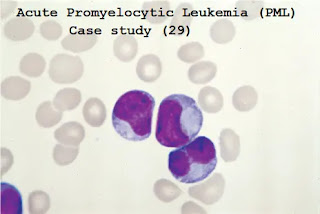
A1. The blood and bone marrow film show leukemic blast cells containing dense granules, many of which have condensed to form Auer rods. Some cells have multiple Auer rods.
This appearance is typical of acute promyelocytic leukemia (PML).
This form of leukemia is associated with a translocation of material from chromosome 15 (PML) to chromosome 17 (at the site of the retinoic acid alpha receptor, RARA) (t15–17).
The figure below shows a variant form of M3 (microgranule variant), where the cytoplasmic granulation is less marked, but the "dumbbell"-shaped nucleus it is characteristic.
Molecular analysis shows a disease-specific fusion gene (PML-RARA).
Cytogenetic and fluorescence in situ hybridization (FISH) changes of acute promyelocytic leukemia (APML) are illustrated in the figure below.
A2. The coagulation changes are consistent with disseminated intravascular coagulation (DIC).
A3. Acute promyelocytic leukemia is complicated by disseminated intravascular coagulation – a well-recognized association.
A4. The coagulopathy frequently gets worse when chemotherapy is commenced.
All-trans retinoic acid (ATRA) is given by mouth and can induce maturation of leukemic cells; its use is associated with a lower incidence of DIC, a higher rate of remission, and improved long-term survival.
Modern chemotherapy regimens for APML focus on three to four courses of anthracycline-based chemotherapy (e.g. idarubicin plus cytosine arabinoside).
Once the PML-RARA transcript cannot be detected, the patient can be relieved and ATRA can be stopped.
Arsenic trioxide is used as the first-line treatment for relapsed patients.
For patients who are not suitable for intensive chemotherapy, it can also be used as a first-line treatment. However, it is cardiotoxic and should be carefully monitored.
Other treatments for recurrent disease include anti-CD33 monoclonal antibody (Myelotarg) or allogeneic stem cell transplantation.










Comments
Post a Comment